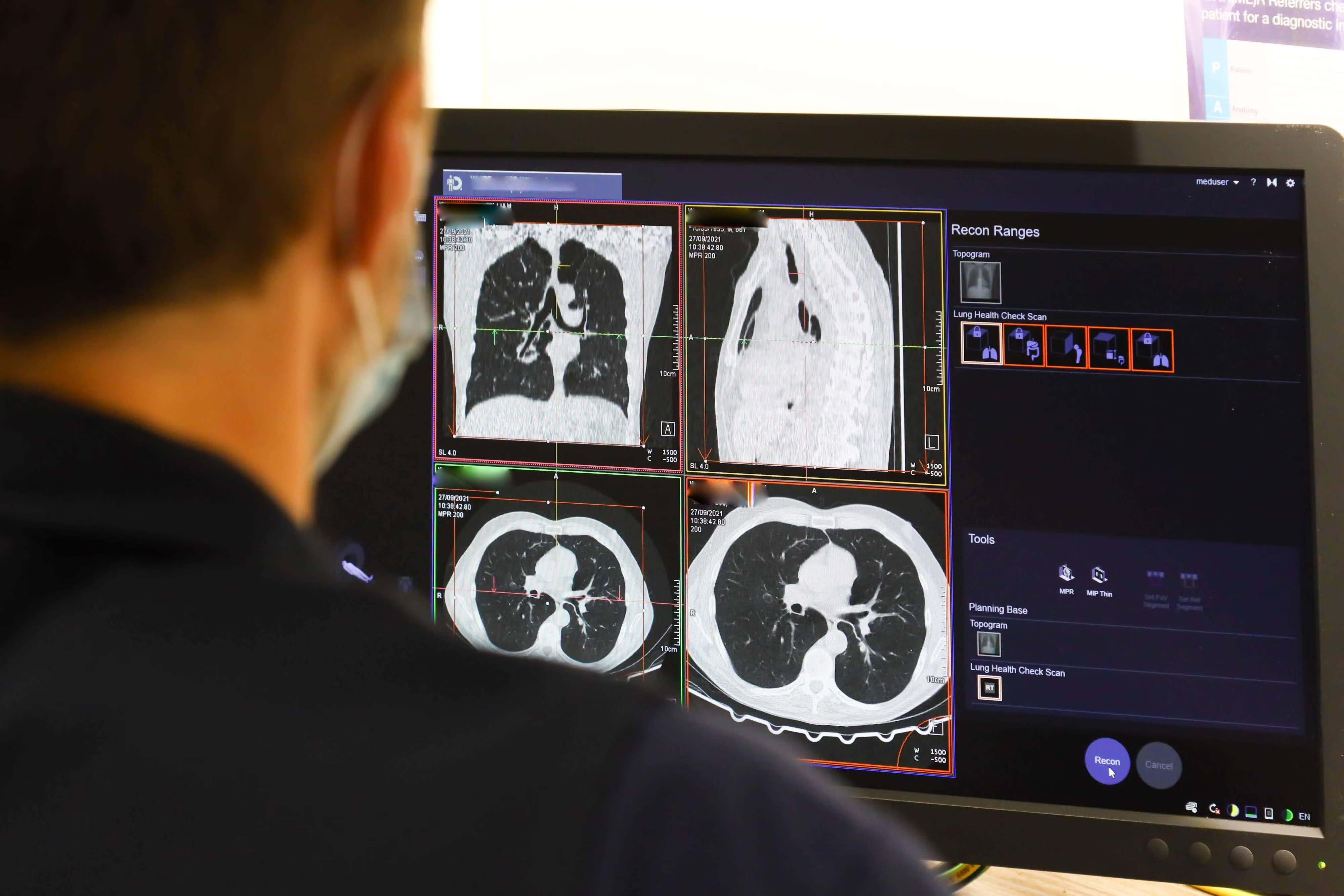

A string of pilot programmes and diagnostic innovations is giving the NHS a practical route to one of its biggest ambitions: faster cancer diagnosis. Early local pilots that use integrated data tools, quicker triage and simplified testing pathways report shorter waits, and some sites have already met the 28-day Faster Diagnosis Standard. Ministers are now backing a wider roll-out of Community Diagnostic Centres (CDCs) and new diagnostic pilots to take those gains nationwide.
Faster Cancer Diagnosis Efforts Accelerate as New Pathways, CDC Expansion and AI Pilots Show Early Promise
National cancer waiting-time data show that England is still missing key targets, including the Faster Diagnosis Standard and the 62-day referral to treatment goal, which means any intervention that reduces diagnostic delay carries immediate clinical value. Faster diagnosis increases treatment options and generally improves outcomes, making diagnostic capacity a health-policy priority.
Several experimental pathways that use a federated data dashboard known as Cancer 360, paired with rapid triage and one-stop diagnostic appointments, have reduced the time from urgent referral to diagnosis in participating trusts. Early evaluations report more timely decisions and better coordination of test sequences, with some sites meeting the 28-day standard during pilot phases. That demonstrates the potential of process redesign and better data use to remove weeks from diagnostic timelines.
Ministers are responding by expanding the places where tests are carried out. The CDC programme, which is already opening hundreds of community sites, has extended operating hours at many centres and is receiving additional capital to increase overall capacity. By delivering more MRI, CT and endoscopy appointments in community settings, the system reduces hospital bottlenecks, offers patients more convenient access to tests and allows acute trusts to focus on more complex care. Recent announcements commit additional funding and hours to scale CDC activity.
New pilots are trialling AI-supported MRI interpretation, including a rapid prostate MRI pathway, alongside liquid-biopsy methods that could identify cancer earlier with fewer appointments. These advances aim to accelerate clinical decision-making and reduce reliance on invasive procedures. However, technology alone cannot solve the problem. Successful roll-out requires robust evaluation, regulatory approval and, most importantly, a workforce of trained radiographers and diagnostic specialists capable of supporting expanded capacity.
Scaling Diagnostic Gains Will Require Workforce Expansion, Better Data Sharing and Sustained Investment
Challenges continue to persist. Pilots typically rely on dedicated teams and targeted funding, but achieving similar results nationwide will require a larger workforce, better data interoperability and a deliberate focus on equity to ensure rural and deprived communities are not left behind. Key measures to monitor in the coming months are the 28-day Faster Diagnosis Standard, 62-day (Referral To Treatment) RTT performance, and diagnostic waits over six weeks (DM01).
The story so far is cautiously optimistic. Pilot programmes demonstrate that smarter pathway design, better data use and stronger local diagnostic capacity can meaningfully reduce waiting times. The Government’s intention to expand CDCs and support emerging diagnostic innovations is a logical next step, yet converting early success into population-wide benefit will depend on sustained investment in staff, modern equipment and interoperable systems. If policymakers secure these foundations, faster diagnosis can shift from a regional trial to a routine feature of NHS care, and for many patients that shift will deliver earlier treatment and, in numerous cases, improved survival.