
Over the past five years we have seen an acceleration of new and innovative therapies in Sickle Cell Disease (SCD). Previously, this condition has experienced significant underinvestment, systemic inequity, and a lack of meaningful therapeutic options. This has been due to several reasons; not withstanding the fact that pharma companies have investors and need to demonstrate that investments in new therapeutic areas will deliver value for patients and meaningful returns for investments. That is finally beginning to change. Multiple new therapies are entering the market, and the momentum behind innovation is real. But if we are going to deliver real change for patients, the evidence frameworks and bodies of evidence used to support these therapies must evolve too.
The reality is this. Current datasets cannot carry the weight of what comes next. National datasets like HES and CPRD provide volume, but they were never built to capture the complexity of SCD. They tell us who was admitted and when, but say little about the pain, VOCs and other factors that led to that admission or the recovery that followed. They are silent on outcomes, quality of life, sleep disturbance, fatigue, depression, and the early signs that many patients recognise long before a crisis hits. These are not peripheral details. These are the outcomes that matter, and they are what define value in the real world.
This disconnect stands in direct contrast to the NHS 10-Year Plan and recent life sciences priorities, which emphasise remote monitoring, personalised care, and reducing preventable economic loss from untreated health needs. With the UK aiming to become one of the top three countries globally for patient access by 2030, particularly in MedTech, remote monitoring, and real-world data, the ability to capture lived experience and early deterioration is no longer optional, it is foundational. Without the right data infrastructure, we risk missing the window to act early, intervene effectively, and reduce the £132 billion in lost GDP tied to unmet health needs. Progress on innovation will stall if the systems that evaluate value remain rooted in incomplete, retrospective views of care.
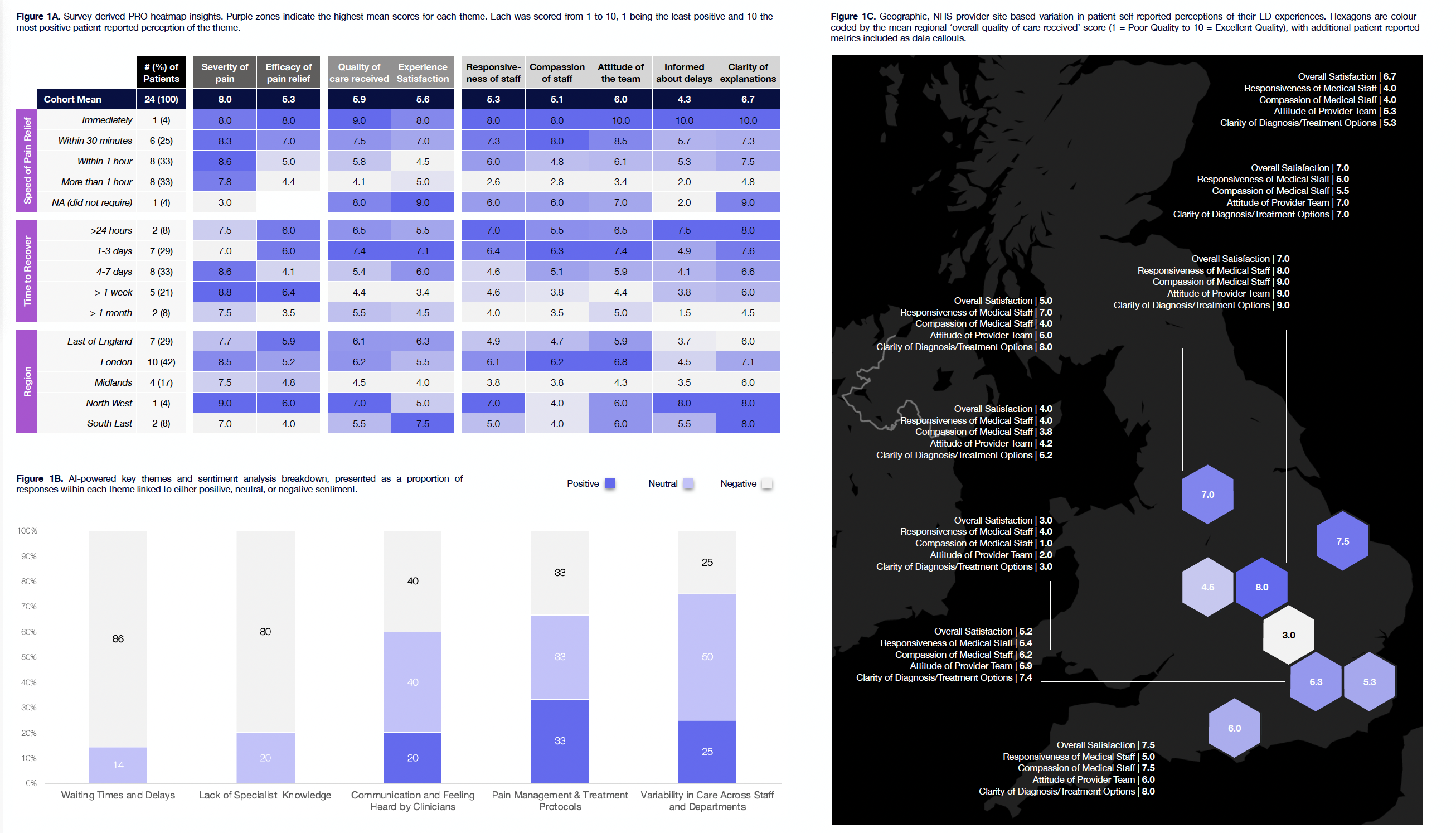
Without a shift in how we collect and apply data, access to these new therapies will be delays in approvals. NICE and other regulators cannot act on signals they cannot see. Commissioners cannot plan services around invisible needs. Industry cannot make the case for therapies without a baseline that reflects the lived reality of patients. And patients themselves remain caught in a system that underestimates both their burden and their potential for better outcomes. With the NHS going through one of its most aggressive and transformational periods on cost containment and budget cuts, ensuring that pharma, patient organisations and regulators have stronger data and evidence for approvals is vital.
We need new infrastructure. That means national, patient-centred registries that go beyond the administrative record. It means longitudinal data that integrates patient-reported outcomes, biometric signals, and real-world use of therapies. It means recognising that the future of equitable access depends not just on developing new treatments, but on building the systems that allow us to properly assess them.
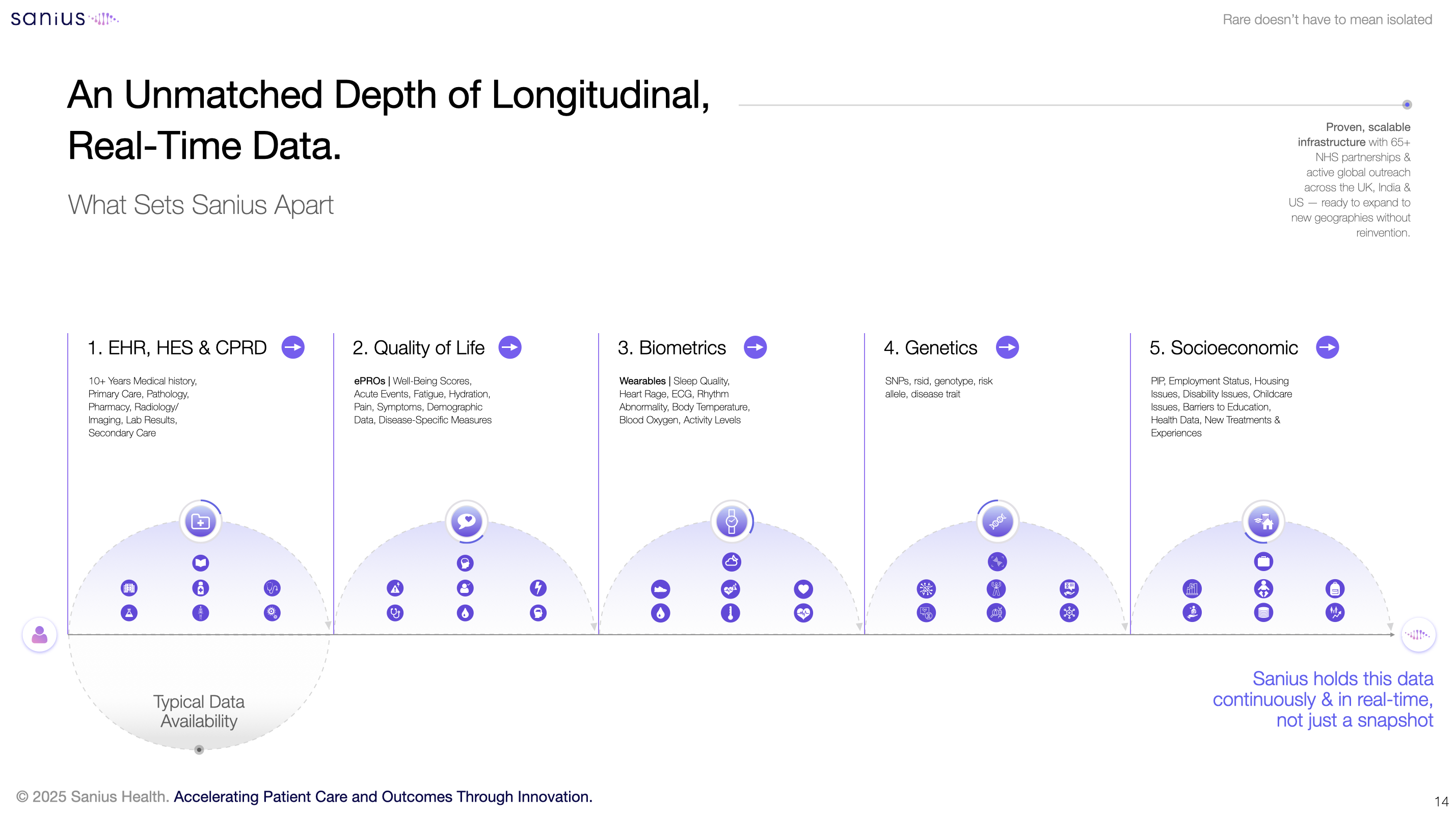
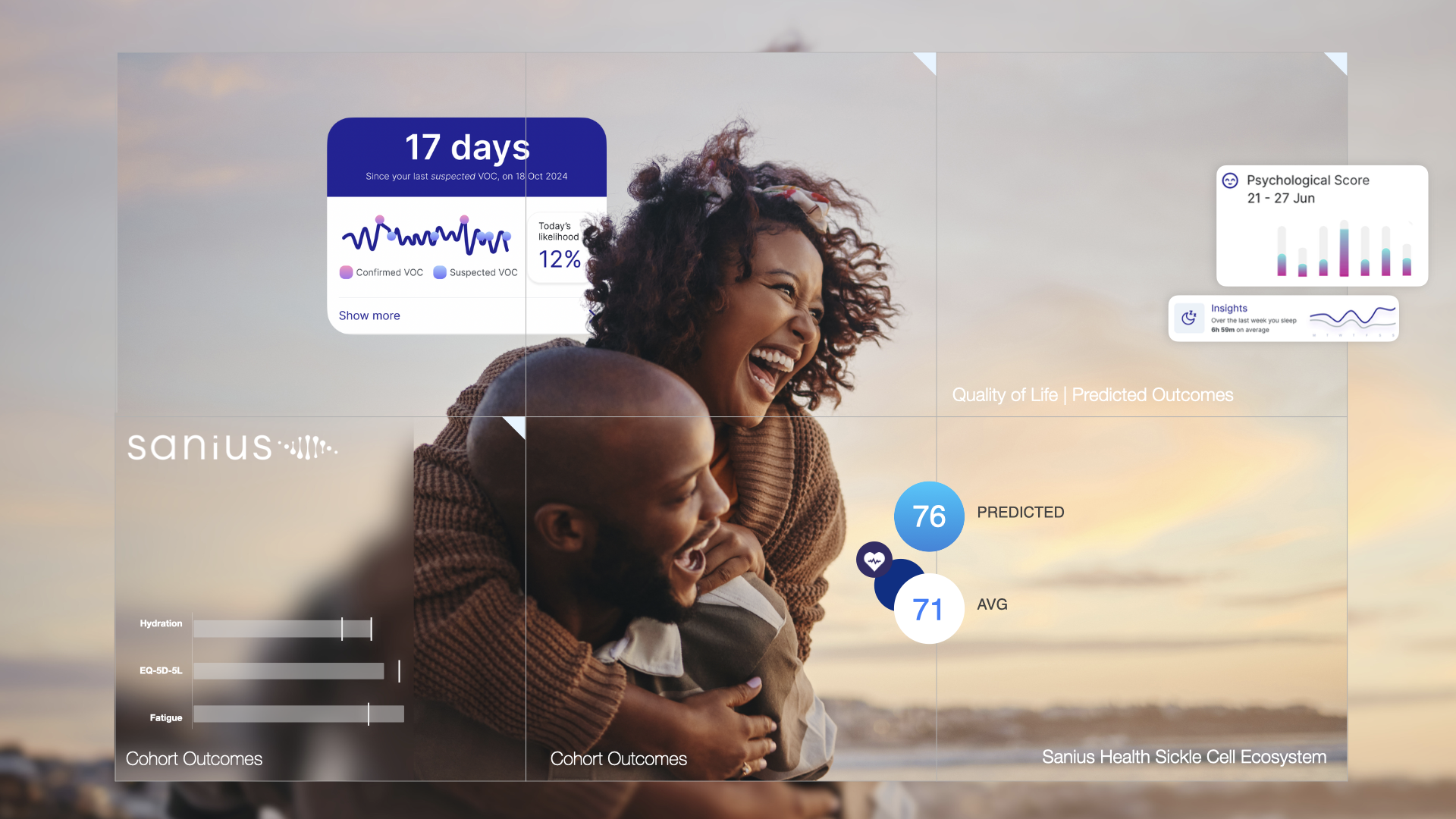
In light of rising demand for SCD therapies, Sanius Health is building this capability as an ecosystem. Through one of the most comprehensive global platforms and registry for SCD, the company combines AI-powered insights, deep correlation, patient-generated data, and clinical-grade monitoring to create a dynamic view of disease progression and treatment impact. The Sanius platform tracks quality of life, symptom burden, and physiological changes across time. It predicts crises using biometric data. It reduces noise in economic modelling by providing a validated, real-world reference point. And it supports more consistent, evidence-based decisions around access and reimbursement.
The organisation is also producing a national-level cost benchmark for SCD that will be made available to NHS bodies, life sciences partners, and policy stakeholders. This work provides a clinically validated pricing index for emergency care, inpatient management, outpatient interventions, and therapy utilisation. It is designed to support fast, equitable decisions and reduce duplication in modelling across the system.
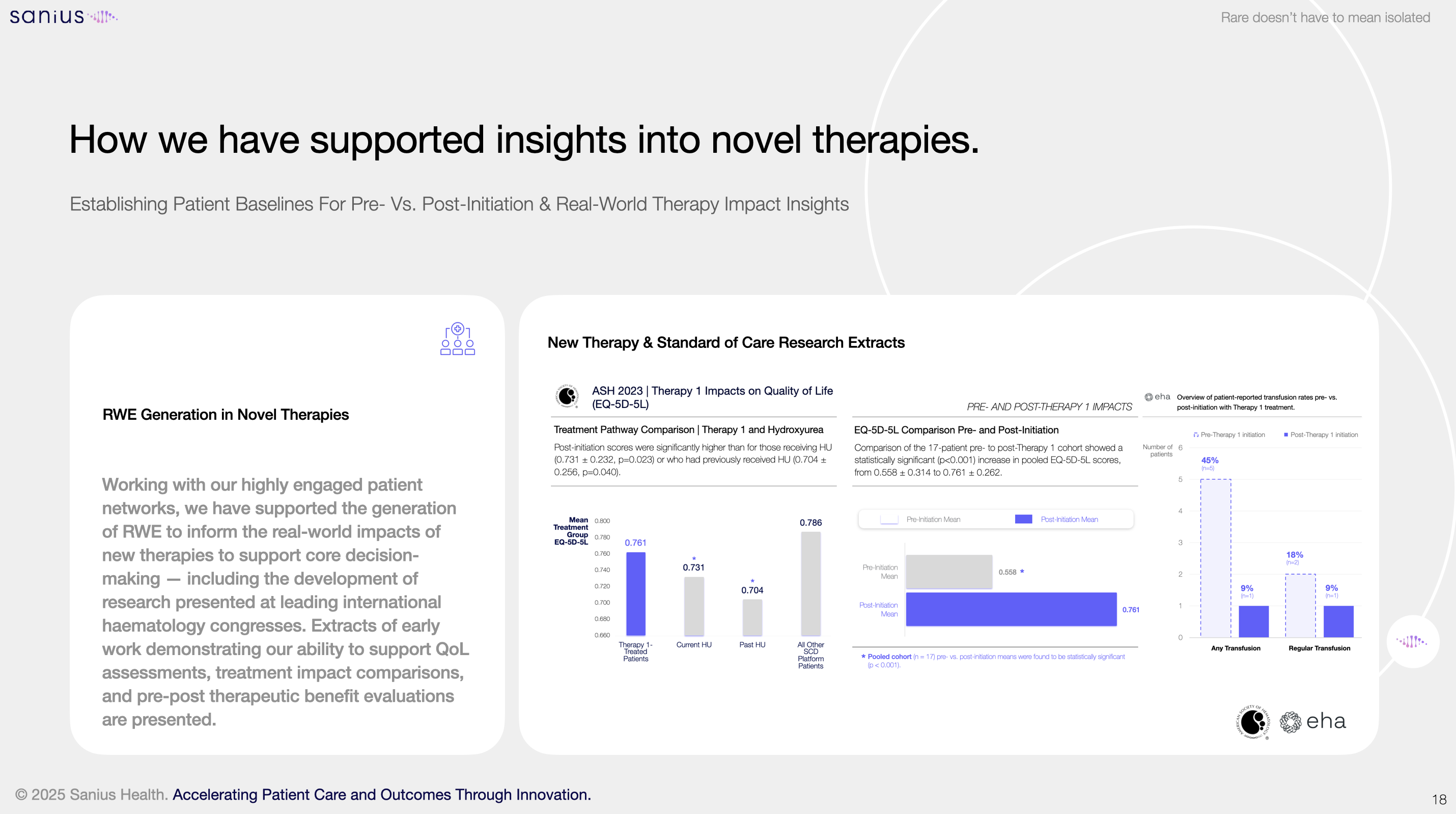
These tools are already in use. Sanius’s insights continue to be used by most therapy developers to demonstrate the unmet need for patients and outcomes. It is being integrated into health economic modelling and HTA submissions: supporting academic partnerships, community engagement, and new models of care.
As new SCD therapies emerge, Sanius Health’s goal is simple: to ensure that evidence keeps pace with innovation, and that decisions about access are grounded in the full picture of what patients experience and what health systems need to deliver. If the UK wants to lead the way in SCD innovation, it must also lead in how it generates and applies real-world evidence. The time for that shift is now. And it will not come from legacy systems alone. It will come from new models of collaboration, new standards of evidence, and a shared commitment to outcomes that actually matter. Sanius Health is proud to be part of that work.
To learn more, visit: www.saniushealth.com or get in touch with with the team at hello@saniushealth.com